Use a torch, mirror, and so forth for verification of cleanliness wherever immediate accessibility of region is impossible.
High-quality control laboratory shall supply the outcome of samples analyzed combined with the Restrict of detection (for rinse along with swab technique) with the analytical method applied to research cleaning validation samples.
One batch of each new products shall be taken for a cleaning verification study with swab sampling only and shall be documented as per the annexure of the cleaning verification protocol.
Observe: In the event the cleaning method is remaining transformed once the failure of the result nevertheless three consecutive cleaning operates ought to be validated using a changed cleaning method.
I would love to enroll in newsletters from Sartorius (Sartorius AG and its affiliated firms) based of my individual pursuits.
The ten ppm standards for cleaning validation is often a broadly approved conventional during the pharmaceutical industry, which stipulates that no more than 10 sections for each million of any solution residue need to be existing on manufacturing products immediately after cleaning.
eleven.2 Each individual circumstance need to be assessed individually. The fashion through which boundaries are founded need to be thoroughly viewed as. In creating residual limitations it might not be sufficient to concentrate only about the principal reactant, mainly because other chemical variants could possibly be more challenging to eliminate.
Manual Cleaning: Handbook cleaning is frequently deemed probably the most complicated method to validate. It involves tactics for instance wiping, sink brushing, and tools brushing.
Adherence to regulatory guidelines: Stay up to date with the newest regulatory necessities and guidelines to make certain compliance.
A] Keeping style: This method shall be adopted, by indicates of apparatus design; it can be done to retain the rinse volume.
This post aims to deliver a radical idea of cleaning validation and its job while in the pharmaceutical website industry, highlighting its vital measures and things to consider in this vital process as well as regulatory guidelines that govern the entire course of action.
Immersion Method: The immersion method may be either agitated, exactly where a cleaning agent in a very method vessel is mechanically stimulated, or static, the place the method vessel is soaked website While using the cleaning agent.
A systematic course of action, cleaning validation is seal of authentication for your cleaning course of action's usefulness. It requires the removing of Filth, germs, micro organism & microbes from surfaces & environments.
The Extractables Simulator from Sartorius is exclusive in the industry. It offers scientifically correct scaling information for Sartorius solutions and assemblies, making it achievable to deliver quantitative data for all products sizes, from advancement to large approach scale.
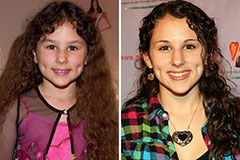 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Jeremy Miller Then & Now!
Jeremy Miller Then & Now! Karyn Parsons Then & Now!
Karyn Parsons Then & Now! Andrew McCarthy Then & Now!
Andrew McCarthy Then & Now! Catherine Bach Then & Now!
Catherine Bach Then & Now!